What effect does it have when the coconut shell is drilled with thousands of holes and used as a lampshade?



Its enchanting lights show a psychedelic romance, and maybe something will be enchanting, and the natural warmth of the coconut shell will overcome the indifference of today's industrial lights.

The designer Vainius Kubilius is located in Lithuania. He hopes that this lamp can be "aphrodisiac for people's lives, because we need enthusiasm". It is called narcissus and makes people feel fascinated.

Narcissus lamps are handmade. Every time a manufacturer drills a hole, he must blow it up to the dust, so it is not too much to say "everything is coming out."


The support base of the lamp, such as the branches of the dragonfly, stretches out a light bulb, which is dynamic and energetic.
The branches are covered with spray paint, which is really not like this texture. Is it the rhythm of wrapping the bandages on the plants? It is good to see when it is used in the night.
Product List
|
aluminium trihydrate (ATH) |
21645-51-2 |
|
magnesium hydroxide (MDH) |
1309-42-8 |
|
antimony trioxide (ATO) |
1309-64-4 |
|
Zinc borate |
1332-07-6 |
|
Melamine polyphosphate (MPP) |
218768-84-4 |
|
Melamine phosphate (MP) |
20208-95-1 |
|
Melamine Cyanurate(MCA) |
37640-57-6 |
|
68333-79-9 |
Introduction
The most common inorganic flame retardants are the hydroxides or aluminium and magnesium. Aluminium trihydroxide (ATH) is by far the most widely used Flame Retardant on a tonnage basis. It is inexpensive, but usually requires higher loadings in polymers of up to more than 60%, because the flame retardant mechanism is based on the release of water which cools and dilutes the flame zone. Magnesium hydroxide (MDH) is used in polymers which have higher processing temperatures, because it is stable up to temperatures of around 300 C versus ATH which decomposes around 200 C.

Fine precipitated ATH and MDH (grain size < 2um) are used in melt compounding and extrusion of thermoplastics like cable PVC or polyolefins for cables. For use in cable, ATH and more often MDH are coated with organic materials to improve their compatibility with the polymer. Coarser ground and air separated grades can be used in liquid resin compounding of thermosets for electrical applications, seats, panels and vehicle parts.
A number of other inorganic substances show flame retarding effects and are used in commercial applications. Most of them are used as synergists i.e. they enhance the performance of other flame retardants or they are used for specific effects like the suppression of smoke formation. For example, borates are used as mixtures of boric acids and borax as flame retardants for cellulose (cotton) and of zinc borate for PVC and other plastics like polyolefins, elastomers, polyamides, or epoxy resins. In halogen-containing systems, zinc borate is used in conjunction with antimony oxide, while in halogen-free systems it is normally used in conjunction with aluminium trihydroxide, magnesium hydroxide, or red phosphorus. In some particular applications zinc borate can be used alone. Boron containing compounds act by stepwise release of water and formation of a glassy coating which protects the surface.
Zinc compounds were initially developed as smoke suppressants for PVC (Zinc hydroxystannate). Later it was found that they also act as flame retardants in certain plastics mainly by promoting char formation.
Intumescent Flame Retardant systems expand to produce foams. They are used as coatings not only to protect combustible materials such as wood and plastics, but also steel structures in buildings, because steel loses its strength when exposed to high temperatures in a fire. The intumescent effect is achieved by combining an acid source like ammonium polyphosphate, a source of carbon, compounds which release noncombustible gases for blowing the foam on thermal decomposition and resin binders to stabilise the foam.
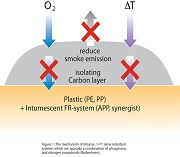
Expandable graphite is manufactured from flake graphite by treatment with strong acids like sulphuric or nitric acid. The acid is trapped in the crystal layers of the graphite ("intercalated"). When it is heated, the graphite starts to expand up to several hundred cm3 per gram, forming a protective layer for the polymer. Expandable graphite is used in plastics, rubbers (elastomers), coatings, textiles and especially in polymeric foams. To achieve an optimum flame retarding effect, the use of synergists like ammonium polyphosphate or zinc borate is often necessary. The black colour of graphite limits its applicability in some cases.
Nanocomposites have been gaining increasing attention since the late 1990s as potential new flame retardants. Nanocomposites
are polymer layered silicates based on aluminosilicate clay minerals like montmorillonite, composed of layers with gaps (gallery spaces) in between. These silicates have the ability to incorporate polymers. Research with nanocomposites has focused on plastics like polymethylmethacrylate (PMMA), polypropylene, polystyrene, and polyamides. Nanocomposites particularly prevent dripping and promote char formation. Therefore, they have been used as synergists in some polymer / flame retardant combinations. However, they require special processing and for the time being are not considered to become viable stand-alone flame retardants.
Other inorganic fillers like talcum or chalk (calcium carbonate) are sometimes denoted as flame retardants, but they do not specifically interact with the ignition process. On the contrary, simply by diluting the combustible polymer they reduce its flammability and fire load.
Inorganic Flame Retardant Additives, Inorganic Flame Retardant Polymers, Inorganic Flame Retardant Chemicals,Ammonium Polyphosphate
Shandong Novista Chemicals Co.,Ltd (Novista Group) , https://www.novistachem.com