 |
Researchers at Berkeley Lab and the Joint Center for Artificial Photosynthesis have proven to convert carbon dioxide recovery into valuable chemicals (such as ethylene and propanol) and fuels (such as ethanol) , Both economical and efficient-all of this can be achieved by specific product active points on a single copper catalyst.
The research was published in the journal Nature Catalysis published on December 17.
For decades, scientists have been looking for effective ways to eliminate excess carbon dioxide emissions from the air and recycle them into products such as renewable fuels. But the process of converting carbon dioxide into useful chemicals is lengthy, expensive, and wasteful, so it is neither economically nor environmentally feasible.
Direction of action
JCAP researcher Joel Ager led the research. He said that when you take a piece of copper metal, it may feel smooth, but at the micro level, the surface is actually uneven. These The bump is what the scientists call the active point. Agger is a scientist in the Department of Materials Science at Berkeley Laboratory and an associate professor in the Department of Materials Science and Engineering at the University of California, Berkeley.
These active points are the magic of electrocatalysis: the electrons on the copper surface interact with carbon dioxide and water to convert them into products such as ethanol fuel; ethylene, the precursor of plastic bags; propanol, a kind of pharmaceutical industry Commonly used substances.
Since the 1980s, people have discovered that carbon can be converted into various useful products. People always think that its active reaction is not product-specific—in other words, you can use copper as a catalyst to make ethanol, ethylene, propanol, Or other carbon-based chemicals, but you have to go through many separation steps, through the residual chemicals formed in the intermediate stage to reach your destination-the final chemical product.
The goal of green or sustainable chemistry is to get the product you want during the chemical synthesis process, Agger said. You do n’t want to spend effort to separate what you do n’t want from the ideal product, because that is both expensive and not environmentally friendly. This cost and waste reduces the economic viability of carbon-based solar fuel.
So when Agger and collaborator Yanwei Lum, a Ph.D. at the University of California, Berkeley, were working together, the students of Agger Lab were studying the copper catalytic performance of solar fuel projects. They wanted to know if, like photosynthesis in nature, Similarly, the electrons in solar cells can be used to drive specific active points of copper catalysts, and whether carbon-based fuels or chemicals can be manufactured, Agger said.
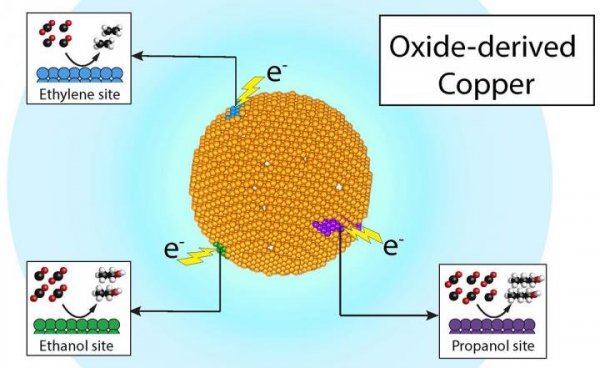
Researchers at the Berkeley Laboratories and the Joint Center for Artificial Photosynthesis have demonstrated that converting carbon dioxide into valuable chemicals (such as ethylene and propanol) and fuels such as ethanol is both economical and efficient-all products can be passed through a single To achieve specific product active points on the copper catalyst. Image source: Joel Ager and Yanwei Lum / Berkeley Lab
Tracking the source of chemicals through passports
Previous research has shown that oxidized or rusty copper is an excellent catalyst for making ethanol, ethylene and propanol. Agger said that the researchers speculate that if the active points on the copper surface are actually product-specific, they can track the source of these chemicals through carbon isotopes, just like the stamp on the passport can tell us which countries have been to.
Ager said: "When we think of this experiment, we think it is a less mainstream idea, if you can try it, it is really crazy. But we can't abandon this idea, we also think it will work because Our previous research on isotopes has allowed us to discover new ways of reacting. "
So in the next few months, Lum and Argel used two isotopes of carbon-carbon 12 and carbon 13-to do a series of experiments as a seal on their passports. Carbon dioxide is labeled with carbon 12, and carbon monoxide, a key intermediate in the formation of carbon-carbon bonds, is labeled with carbon 13. Based on their method, the researchers concluded that the ratio of carbon-13 to carbon-12 found in a product will determine which active site the chemical product originated from.
Lum conducted dozens of experiments and analyzed the results at JCAP using state-of-the-art mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy. They found that three of these products—ethylene, ethanol, and propanol—have different isotopic characteristics, indicating They come from different locations of the catalyst. Lum said the discovery inspired future work to isolate and identify these different active points. Lum said that putting these product-specific active points into a single catalyst will one day produce chemical products very efficiently and selectively.
Chemical manufacturing will usher in a greener day
The researchers ’new method — described by Agger as a simple chemical environment and economic turning point — is the new beginning of their desired green chemical manufacturing, in which solar cells can feed electrons to specific active points on copper catalysts to Optimize ethanol fuel production.
He said that maybe one day, this technology could make facilities like the "Sunlight Refinery" possible, driven by solar energy, extracting carbon dioxide from the atmosphere and creating a series of useful products.
(Originally from: Daily Science China New Energy Network Synthesis)
Purification Plate Processing,Clean Room Color Steel Plate,Food Coloured Steel Plate,Process Sandwich Plate
Jiangmen Leonard Industrial Machineries Co., Ltd. , https://www.lndcleanroom.com