For a long time, the cruising range is the bottleneck restricting the development of electric vehicles. We call this process anxiety. Improving the cruising range on the one hand has to increase the capacity of the battery pack, but more importantly, it increases the specific energy of the battery. At present, the weight-to-energy ratio of the ternary material lithium-ion power battery is generally 200 Wh/kg, and the technology continues to improve. It is expected that the production of high specific energy power batteries with a specific power of 300Wh/kg will be launched in 2020, but this still cannot meet the demand for the future development of electric vehicles. With regard to the development of the next generation of high specific power batteries, there are currently several routes to choose from. One is all-solid-state lithium metal batteries, which is a widely accepted and recognized technology route. The US’s “Battery 500†program is Through the development of lithium metal secondary battery technology to achieve the goal of achieving a specific energy of 500Wh/kg, Japan's solid electrolyte technology is at the leading level in the world, and its developed ionic conductivity of sulfide electrolytes can even be comparable to liquid electrolytes; One technical route is metal-air batteries, such as the current mainstream Li-air and Na-air batteries, whose theoretical specific energy exceeds 2000Wh/kg, much higher than lithium-ion power batteries.
Recently, Qichen Wang of Central South University and National University of Defense Technology prepared a Zn-air battery using an N-doped graphene material NDGs-800 as an air electrode catalyst. The large number of defects in graphene oxide GO improves the catalytic efficiency of O2 at the air electrode. The performance of Zn-air batteries has been greatly improved, and the specific energy is as high as 872.3 Wh/kg. It has a very broad application prospect in the field of energy storage in the future.
The key to the metal-air battery is the design of the air electrode. The air electrode must have the catalytic O2 reduction and oxygen evolution reaction. The common oxygen electrodes are precious metal (Pt) and rare earth metal oxides, but they are very difficult. Take into account the O2 reduction and oxygen evolution reactions. So people pay attention to the carbon electrode. Research shows that the defects and porous structure in the carbon material can provide many active sites for the reduction and oxygen evolution of O2 in the electrode, thus improving the metal-air battery performance. The redox graphene is just such a very good option, redox graphene itself has a lot of defects, Qichen Wang has introduced more defects in the graphene by N doping means, while graphene is huge The specific surface area and porous structure also provide numerous active sites for the reduction and oxygen evolution of O2, thereby significantly improving the performance of Zn-air batteries.

The method for synthesizing N-doped graphene is shown in Fig. a above. First, a certain amount of g-C3N4 flakes are added to the aqueous solution of graphene oxide GO, sonicated for 1 h, and then the mixed solution is hydrothermally treated at 180° C. for 12 h. The black mixed gel was then freeze-dried for 48 h to remove H2O. The dried material was heated in a tubular furnace under N2 protection to 600-900° C. and heat-treated for 3 h to obtain an N-doped graphene material NDGs-x (x represents the processing temperature).
The structure of N-doped graphene is shown in the figure above, and it can be seen from figures b and c that it has an open-cell structure and typical graphene characteristics. Atomic force microscopy (above figure e) shows that the thickness of graphene is 3 nm, about 9 The layer of carbon atoms is composed of layers. At the same time, the material has a very large specific surface area (443.2m2/g) and a micropore volume ratio (3.43cm3/g), which can provide a large number of active sites for O2 reduction and oxygen evolution reaction.
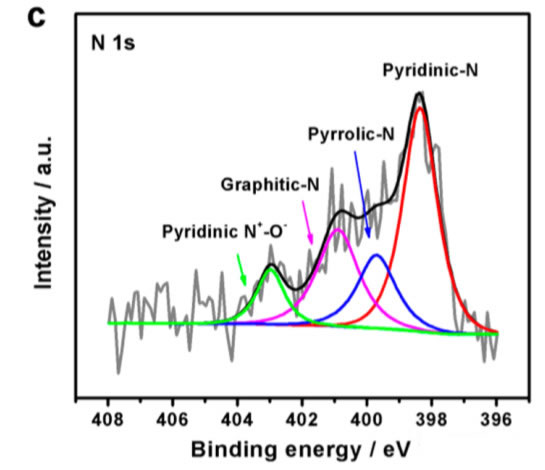
XPS studies have shown that N elements mainly exist in graphene oxide in three forms: pyridine N, pyrrole N, graphite N, and pyridine N+-O-. NDGs sintered at 800°C can be noticed from the following figure d. 800 material has the highest pyridine N content, reaching 47.9%. The widespread presence of such high contents of pyridine N and redox graphene GO significantly promotes the efficiency of catalytic O2 reduction and oxygen evolution reactions.

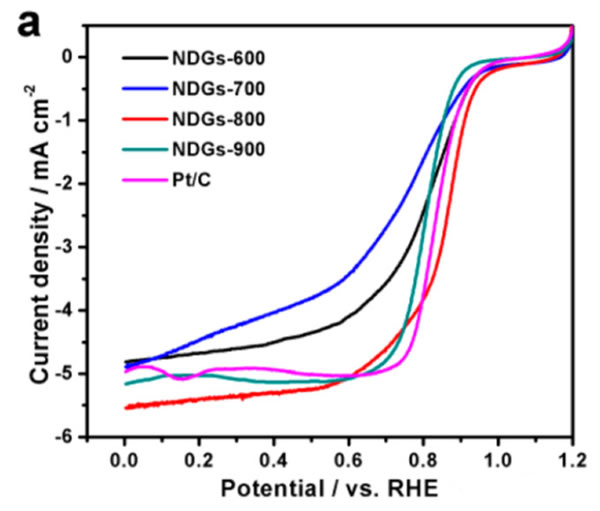
More reactive sites helped the N-doped graphene NDGs material to obtain better reactivity. From the linear voltage sweep of the following figure a, it can be seen that the NDGs-800 material (red curve) shows a very high catalytic O2 reduction. Reactivity, the initial reaction voltage was 0.95 V, the half-wave voltage also reached 0.85 V, and the reaction current density at 0 V reached 5.6 mA/cm2. From the figure b below, we can note that the reaction current density (13.91mA/cm2) of the NDGs-800 at 0.8V is even higher than the reaction current density (13.32mA/cm2) of the Pt/C composite catalyst, which is much higher than that of the NDGs. -900 (6.03 mA/cm2), NDGs-600 (55.55 mA/cm2), and NDSs-700 (2.80 mA/cm2), making the NDGs-800 material the best non-metal reaction catalyst.

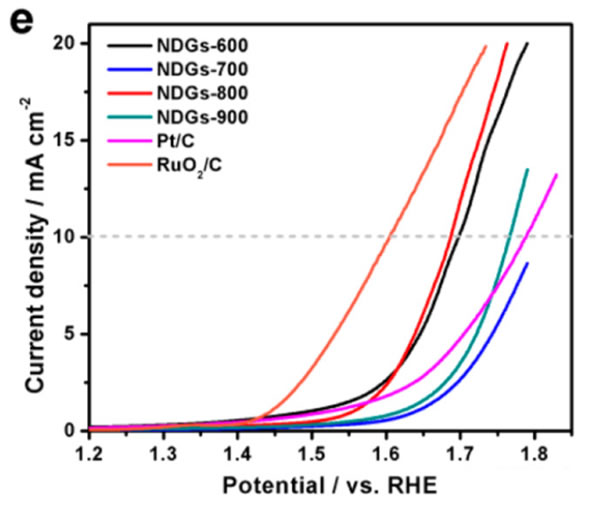
Although the activity of NDGs-800 catalysed O2 reduction reaction is very high, we still need to investigate the activity of NDGs-800 catalytic oxygen evolution reaction. From the figure below we can see that NDGs-800 material is analyzed at a current density of 10 mA/cm2. The overpotential of oxygen reaction is 375mV higher than that of RuO2/C catalyst, indicating that the catalytic efficiency of oxygen evolution reaction of NDGs-800 material is not as good as that of RuO2/C catalyst. This is where NDGs-800 materials need to be improved in subsequent research.
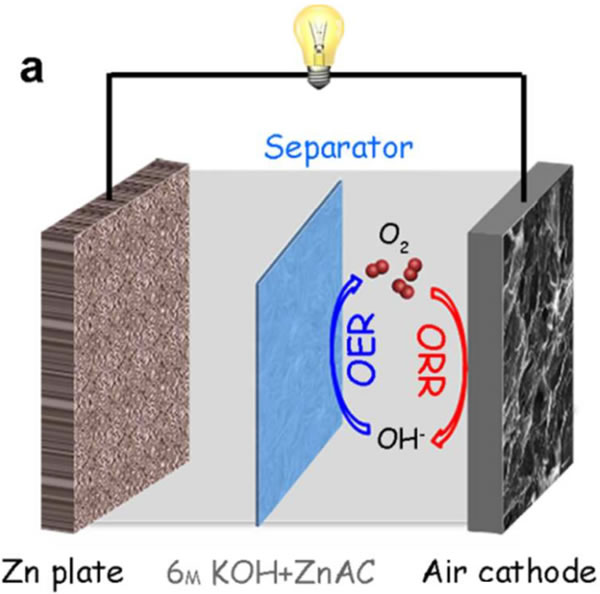
Qichen Wang uses a combination of NDGs-800 material Zn-air battery (structure shown below), the battery's open rate voltage is 1.45V, power density is 115.2mW/cm2, better than Pt / C catalyst (1.43V, 110.3 mW/cm2), the specific capacity of the Zn anode using the NDGs-800 material reaches 750.8 mAh/g (current density 10 mA/cm2), and the specific energy of the battery reaches 872.3 Wh/kg. The battery also exhibited excellent cycle performance. At a current density of 10 mA/cm2, the cycle of 234 cycles (20 min per cycle) showed almost no decline in the cell, much better than the Pt/C+Ir/C catalyst. Battery.
The N-doped graphene material NGDs-800 material developed by Qichen Wang took full advantage of a large number of defects in graphene oxide GO, and introduced more defects through N doping, providing a large number of O2 reduction and oxygen evolution reaction catalysis The active point greatly improves the catalytic efficiency, especially in catalytic O2 reduction, its catalytic efficiency is even higher than that of Pt/C electrode, and it also shows excellent stability in the charge-discharge cycle, and has broad application prospects. However, the activity of catalyzing oxygen evolution reaction of NDGs-800 is still not as good as that of RuO2/C catalyst, which is also the place where improvement is needed.
First, the impact resistance - its structure, the panel has a strong resistance, this feature is based on the BSEN438-2/91 sports sphere sag test results, and confirmed by daily practical use.
Second, resistance to scratching - special surface structure, so that the ceramic plate has scratch resistance, even if it is affected by various hard objects, it can maintain the shape without damage for a long time.
Third, wear-resistant - BSEN438-2/91 test, confirmed that the ceramic plate has a strong wear resistance, suitable for heavy objects placed or need frequent cleaning.
Fourth, easy to clean - tight non-permeable surface, so that dust is not easy to adhere to it, so the product can be easily cleaned with the relevant solvent, without any impact on the color.
Alumina Ceramic Disc,Wear Resistance Alumina Ceramic,Ceramic Zirconia Alumina Abrasive,Alumina Ceramic Crucible
SHENZHEN HARD PRECISION CERAMIC CO.,LTD , https://www.hardcm.com